The clinical trial landscape continues to evolve rapidly, with new developments in drug approvals, study designs, and regulatory frameworks. This update provides a snapshot of the latest advancements in clinical research as of March 17, 2025.
Breakthrough in Alzheimer's Treatment
Alzheimer’s Drug (Phase III): Early data indicate a 30% reduction in cognitive decline. This Phase III trial, conducted by NeuroPharma, demonstrated a significant slowing of disease progression in patients with early-stage Alzheimer's. This development could potentially change the landscape of Alzheimer's care and provide hope to millions of patients and their families.
Vaccine Trial Updates
Several new COVID-19 vaccine trials have commenced this month, focusing on next-generation vaccines designed to tackle emerging variants. Notably, BioVaxCorp launched a Phase II trial for its multivalent vaccine, which aims to provide broader protection against multiple strains of the virus.
The U.S. FDA placed BioNTech’s malaria vaccine study on clinical hold. The U.S. Food and Drug Administration has placed a clinical hold on BioNTech's early-to-mid stage trial of an experimental malaria vaccine, the drugmaker said in a filing. (Reuters)
HIV Treatment Advances
Gilead Sciences, Inc. (Nasdaq: GILD) has unveiled groundbreaking data and several oral presentations from its cutting-edge HIV treatment portfolio at the Conference on Retroviruses and Opportunistic Infections (CROI 2025). The findings underscore the company's innovative approach and swiftly progressing pipeline aimed at expanding treatment options and improving outcomes for individuals living with HIV, while also striving towards a cure. (Gilead)
Advances in Cancer Immunotherapy
In the realm of cancer research, a novel immunotherapy approach has shown remarkable efficacy in treating metastatic melanoma. The study, led by OncoImmuno Inc., reported a 70% response rate in patients who had previously exhausted other treatment options. These findings have sparked optimism for the future of cancer immunotherapy.
Regulatory Changes and New Guidelines
The FDA has issued final guidance on decentralized clinical trials (DCTs), addressing key issues such as data variability and investigator oversight, aiming to make the process more patient-centric and efficient. These changes are expected to facilitate greater participation and streamline trial operations, ultimately accelerating the development of new therapies. ( FDA Guidance)
The EMA has adopted the E6(R3) Good Clinical Practice guidelines, effective July 23, 2025, aligning with international standards for clinical trials. (EMA Recommendations)
Expansion of the Middle East and North Africa Clinical Trial Market
The "Middle East and North Africa Clinical Trial Infrastructure Market Report 2025" indicates that recent regulatory reforms have facilitated more expedient approvals, while the growth of public-private partnerships has enhanced research capabilities.
This report presents a comprehensive analysis of the clinical trial infrastructure markets within the Middle East and North Africa (MENA) region. It offers an in-depth examination of market dynamics and provides a thorough understanding of the MENA clinical trial landscape, categorized by study status, phase, type, results, and sponsor type. (Research and Markets)
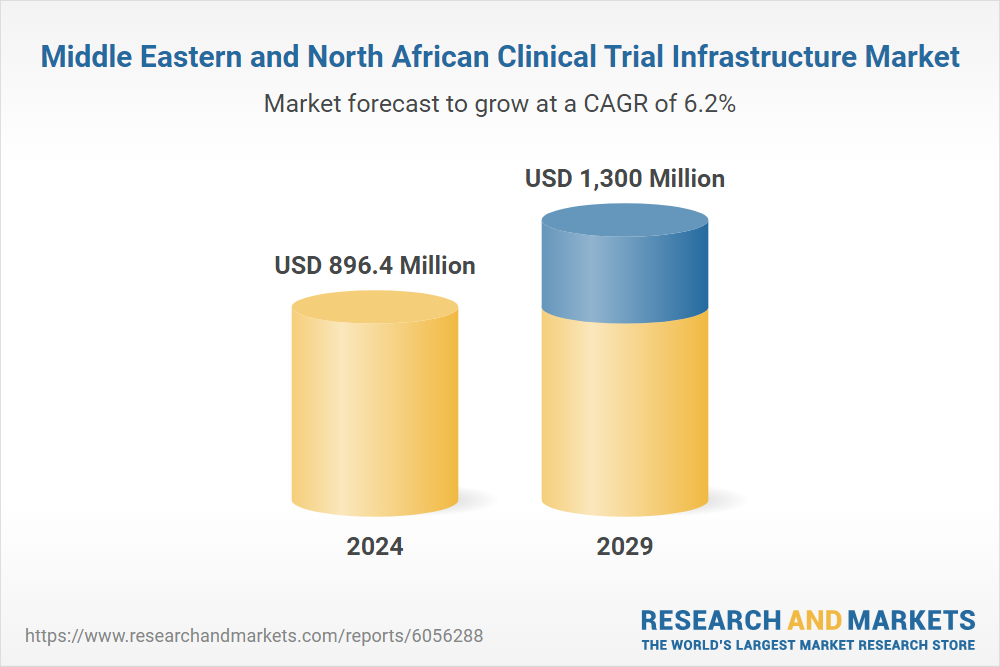
Figure 1. Middle Eastern and North African Clinical Trial Infrastructure Market
Source Links
| Topic | Link |
|---|---|
| FDA Guidelines for DCTs |
https://www.fda.gov/regulatory-information/search-fda-guidance-documents/conducting-clinical-trials-decentralized-elements |
| EMA Recommendation Paper on DCTs | https://health.ec.europa.eu/system/files/2023-03/mp_decentralised-elements_clinical-trials_rec_en.pdf |
| Gilead HIV Treatment News Release 12Mar2025 | https://www.gilead.com/news/news-details/2025/gilead-presents-new-hiv-treatment-and-cure-research-data-at-croi-2025-including-an-investigational-long-acting-twice-yearly-therapy-option |
Conclusion
The clinical trial landscape continues to evolve rapidly, with each month bringing new and exciting developments. March 2025 has proven to be a significant period for advancements in various medical fields, and these breakthroughs underscore the importance of ongoing research and innovation in improving patient outcomes. Stay tuned for more updates as we continue to track the progress of these and other critical trials.
No comments:
Post a Comment